
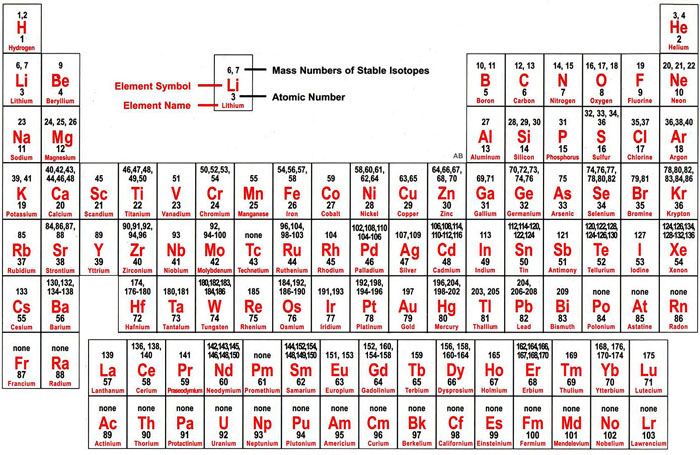
In the third period the 3 s subshell is filling for Na and Mg, and therefore Al, Si, P, S, Cl, and Ar. Across the second period Li and Be have distinguishing electrons in the 2 s subshell, and electrons are being added to the 2 p subshell in the atoms from B to Ne. In the first period the distinguishing electrons for H and He are in the 1 s subshell. The first three horizontal rows or periods in the modern periodic table consist entirely of representative elements. Formulas for chlorides of the first dozen elements that show the periodic variation of valence Element This agrees with the valence rules derived from the periodic table, and results in formulas for chlorides of the first dozen elements that show the periodic variation of valence. For representative elements the number of valence electrons is the same as the periodic group number, and the number needed to match the next noble-gas configuration is 8 minus the group number. That is, the valences of the representative elements may be predicted on the basis of the number of valence electrons they have, or from the number of electrons that would have to be added in order to attain the same electron configuration as an atom of a noble gas. Many of the chemical properties of the representative elements can be explained on the basis of Lewis diagrams. Most of the elements whose chemistry and valence we have discussed so far fall into this category. The representative elements are those in which the distinguishing electron enter an s or p subshell. The type of subshell ( s, p, d, f)into which the distinguishing electron is placed is very closely related to the chemical behavior of an element and gives rise to the classification shown by the color-coding on the periodic table seen here. This last electron is called the distinguishing electron because it distinguishes an atom from the one immediately preceding it in the periodic table. Since it is the outermost (valence) electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in the building-up process is of far more interest to a chemist than the first. The commonly used long form of the periodic table is designed to emphasize electron configurations.


 0 kommentar(er)
0 kommentar(er)
